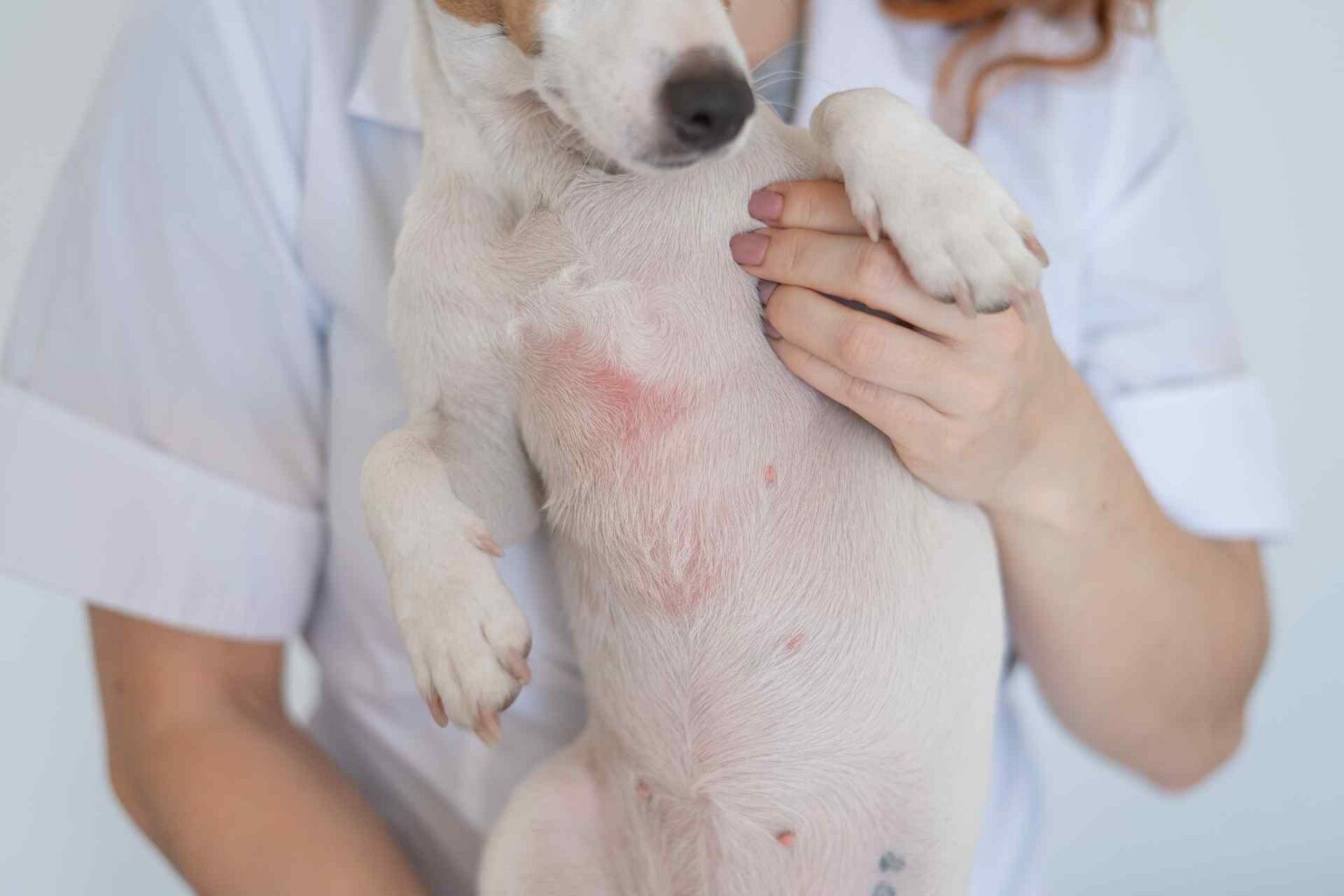
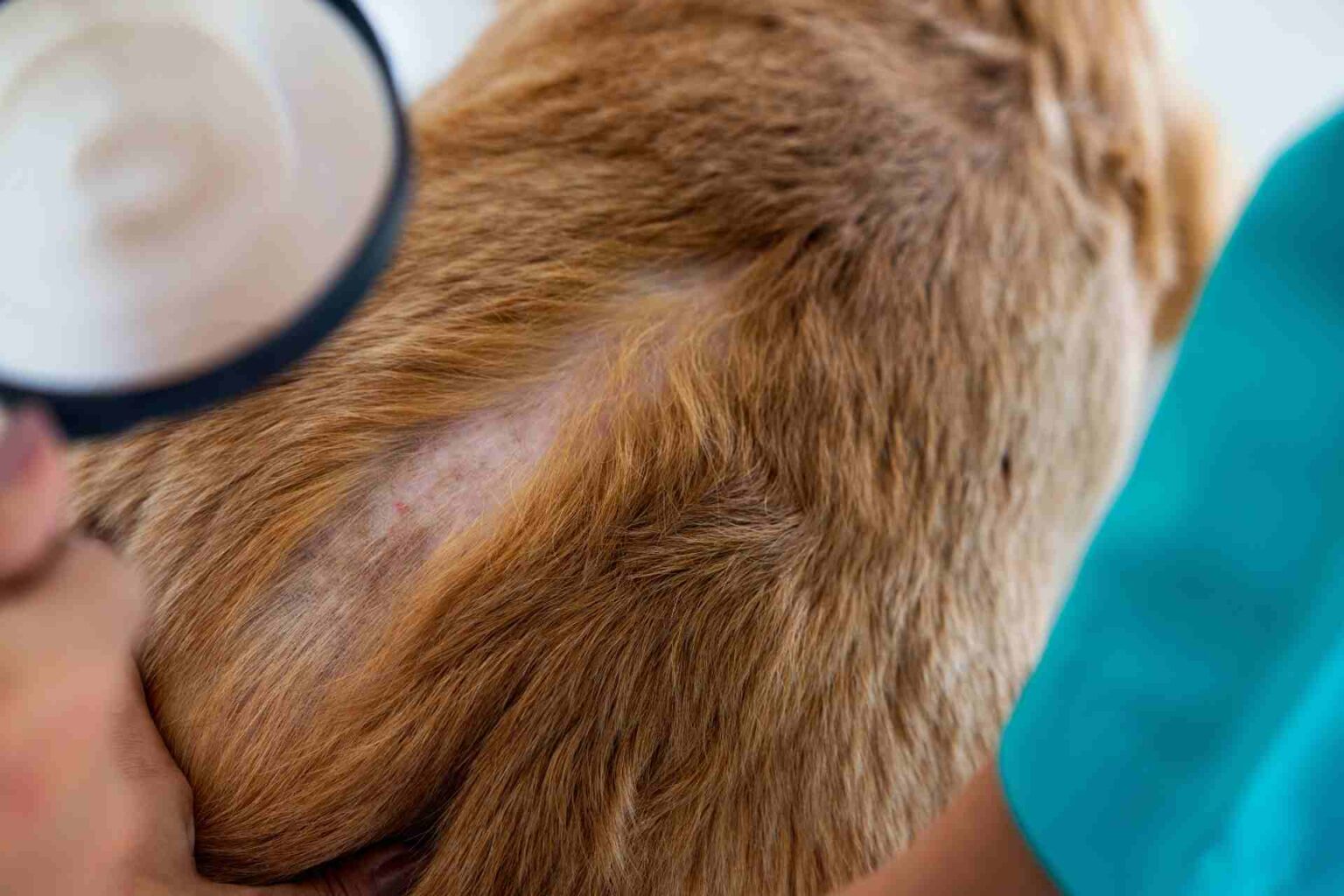
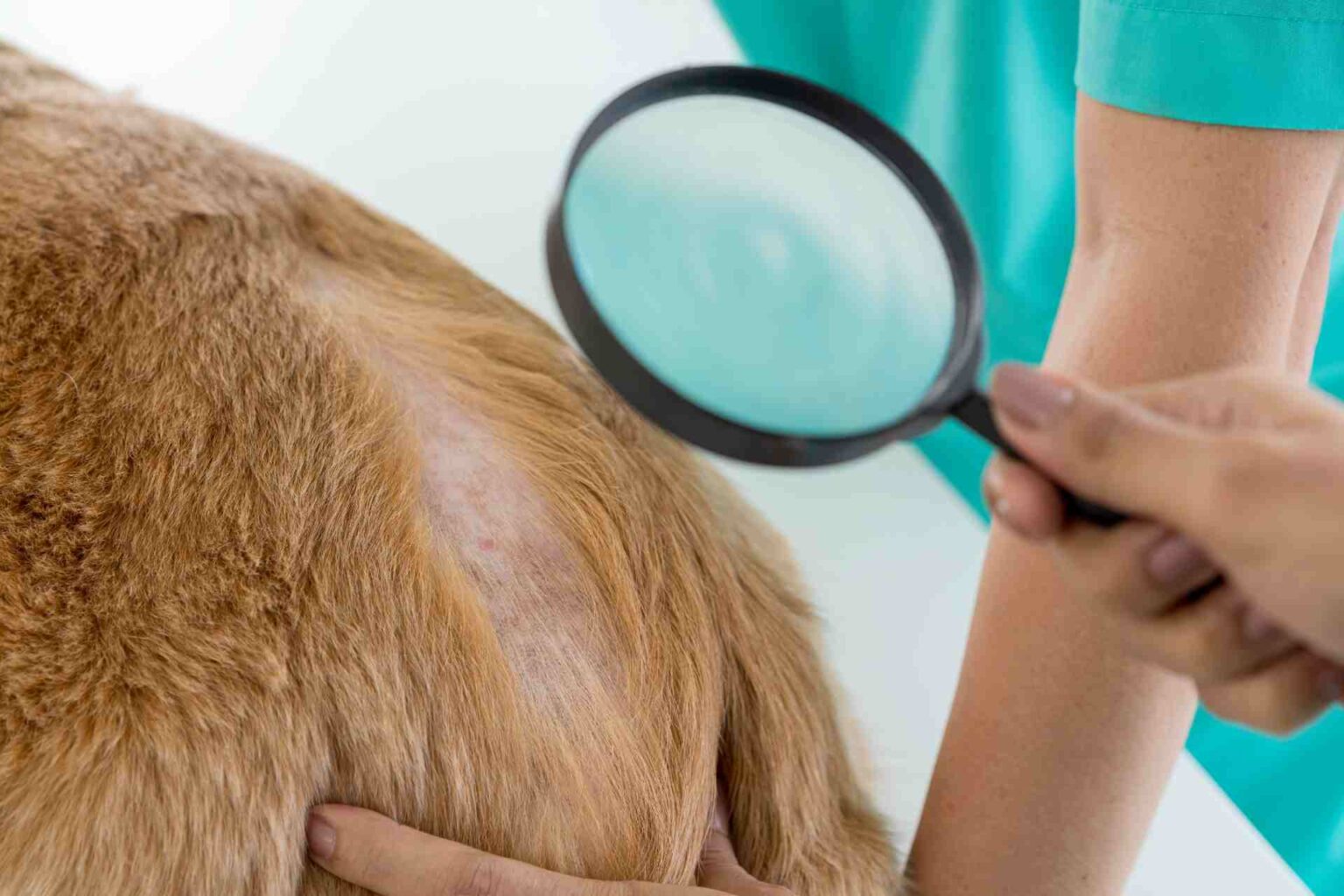
It is Japanese the first study that evaluated in dog atopic dermatitis oclacitinib systemically and hydrocortisone aceponate 0.0584% topically in the first 4 weeks of treatment.
Oclacitinib is a non-selective Janus kinase (JAK) inhibitor for the systemic treatment of canine atopic dermatitis with comparable efficacy to systemic prednisolone and cyclosporine. The most frequent adverse event is gastrointestinal complaints such as diarrhea and vomiting. While the efficacy of oclacitinib monotherapy has been established by several clinical trials, data are scarce on the effects of concomitant topical therapy. Therefore, a Japanese study published in Veterinary Dermatology tested in dogs with atopic dermatitis the efficacy and safety of a combined systemic and topical therapy of oclacitinib and 0.0584% hydrocortisone aceponate (HCA) spray.
The two preparations at the center of the trial
So on the one hand we have oclacitinib, whose most frequent mild adverse event is gastrointestinal complaints such as diarrhea and vomiting.
On the other is 0.0584% hydrocortisone aceponate in a spray formulation: a potent topical corticosteroid that in dogs with atopic dermatitis has been shown to significantly reduce skin lesions within 14 days without serious adverse events. Its efficacy has also been validated for long-term proactive maintenance therapy, in administration on two consecutive days per week regardless of the presence of overt lesions.
Materials, methods and results
The Japanese study- randomized double-blind, placebo-controlled-involved 18 dogs with chronic atopic dermatitis. All were treated with oclacitinib (0.4-0.6 mg/kg) twice daily for 14 days, then once daily for the next 14 days and were then randomized to receive HCA spray or placebo spray, once daily for 7 days and then every other day until the end of the study (day 28).
For clinical evaluation, the CADESI-4 index for symptom severity and the VAS scale for pruritus were applied every 7 days, and blood and urine tests were performed every 14 days.
These results:
- CADESI-4 and VAS scores decreased significantly at day 7 and day 14 in both groups, but from day 14 to day 21 they increased in the placebo group
- most importantly, compared with baseline values, the mean reduction in the HCA-treated group was more significant than that in the placebo group at both day 21 (59.9% vs. 27.6%) and day 28 (56.0% vs. 30.5%).
Data show that topical application of HCA spray is effective in preventing itch flare-ups and injury, particularly when reducing the frequency of oclacitinib administrations from twice to once a day and more effective than oclacitinib as monotherapy during the initial four weeks of therapy.
The combination treatment, continued for four weeks, was well tolerated: no secondary infections or other adverse events were noted.
Despite the objectively low number of dogs involved, the Japanese study is the first controlled clinical trial conducted this combination therapy of a topical corticosteroid and a systemic nonselective Janus kinase (JAK) inhibitor in canine atopic dermatitis.
Reference
Takahashi J, Kanda S, Imanishi I, et al. Efficacy and safety of 0.0584% hydrocortisone aceponate topical spray and systemic oclacitinib combination therapy in dogs with atopic dermatitis: a randomized, double-blinded, placebo-controlled trial. Veterinary Dermatology. 2020 Nov. DOI: 10.1111/vde.12909.